Page 18 - 2021 Spring CMTA Report - Special Research Edition
P. 18
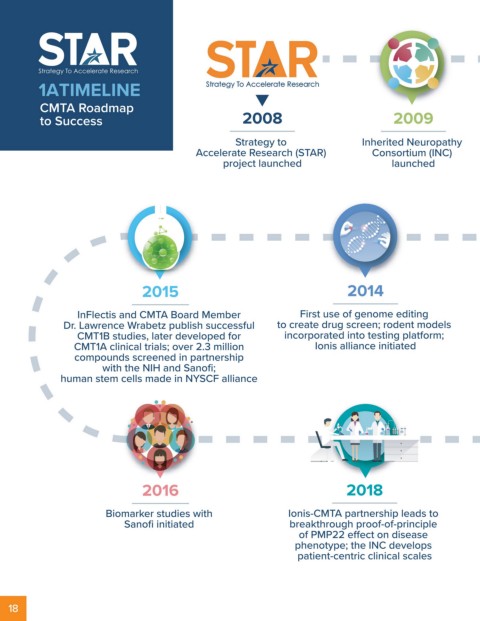
1A TIMELINE Strategy To Accelerate Research
Strategy To Accelerate Research
CMTA Roadmap
to Success 2008 2009 2010 2011
Strategy to Inherited Neuropathy Development of first drug First NIH funding for
Accelerate Research (STAR) Consortium (INC) screening assays in partnership small molecule screening project
project launched launched with the NIH
2015 2014 2013 2012
InFlectis and CMTA Board Member First use of genome editing First clinical trial in USA published First drug screen published;
Dr. Lawrence Wrabetz publish successful to create drug screen; rodent models (Lewis et al., JAMA Neurology); first stem cell project initiated;
CMT1B studies, later developed for incorporated into testing platform; partnership with Sanofi initiated CMT Pediatric Scale published
CMT1A clinical trials; over 2.3 million Ionis alliance initiated
compounds screened in partnership
with the NIH and Sanofi;
human stem cells made in NYSCF alliance
2016 2018 2019 2020
Biomarker studies with Ionis-CMTA partnership leads to Stem cell studies published, Sanofi partnership identifies
Sanofi initiated breakthrough proof-of-principle first gene modifier and skin biopsy assay novel blood biomarker; natural history
of PMP22 effect on disease (treatment-responsive for CMT1A); study published (>1000 patients);
phenotype; the INC develops CMT Health Index (CMT-HI) and gene therapy project with three
patient-centric clinical scales CMT Functional Outcome Scale major institutions launched;
(CMT FOM) published CMTA engaged in pre-clinical testing
or clinical planning with a record
10 biotech partners
18